The first comprehensive clinical trial around the world with our68Ga- and 177Lu-labeled theranostics pharmaceuticals based on homodimer FAP inhibitors is successfully published in Jan. 2022.
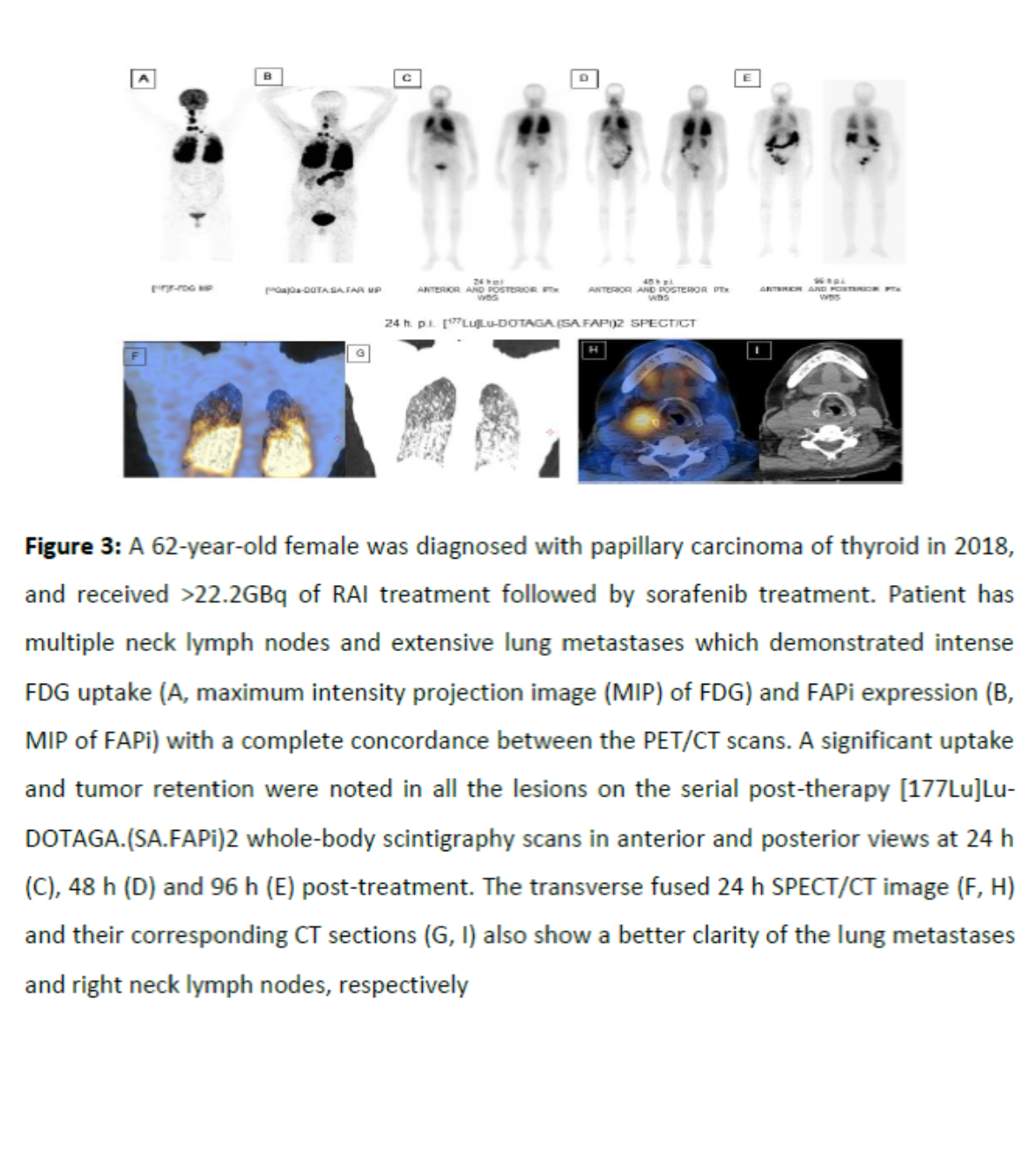
In this trial 15 radioiodine-refractory differentiated thyroid cancer (RR-DTC) patients were recruited, who had progressed on tyrosine kinase inhibitors (TKI). If [68Ga]Ga-DOTA.SA.FAPi PET/CT scan demonstrated moderate-to-excellent uptake in metastases, and patients given informed consents, they received intravenous [177Lu]Lu-DOTAGA.(SA.FAPi)2 as therapy at eight-weekly intervals. The primary endpoints were thyroglobulin (Tg) response, and functional imaging response. Secondary endpoints were visual analog score (VAS) and Eastern Cooperative Oncology Group performance status (ECOG). The ratio of absorbed radioactive doses between tumor lesions and normal organs was extremely high. The Serum thyroglobulin level significantly decreased after treatment vs. at the time of assessment. Patients found significantly less pain and had improved ECOG performance scores after treatment. None of the patients experienced grade III/IV hematological, renal or hepatotoxicity.
This preliminary data suggest our novel molecule [177Lu]Lu-DOTAGA.(SA.FAPi)2 is safe; seems effective, and most importantly opens up a new avenue for the treatment of aggressive RR-DTC patients who have exhausted all standard line of treatments. Therefore a large-scale dosimetry study has been planed and will be launched in upcoming months.